Guaranteeing the Security of Teeth-Cleaning Tools Through Rigorous Testing by the FDA
Ensuring dental burs are safe and effective is critical when it comes to dental health, for which the Food and Drug Administration (FDA) has created a set of regulations. These regulations provide a framework for the manufacture and labeling of dental burs to ensure patient security during dental operations. By doing so, they guarantee that any dental instruments used are up to precise standards.
Under the oversight of the FDA, the process of manufacturing dental burs is kept heavily regulated, from drawing up plans for their design, to using the appropriate materials in their assembly. With these standards enforced, dentists can trust that the resulting product will be safe and effective in its function. Furthermore, relevant labelling is mandated to help practitioners decide on which bur would be best-suited for each task.
To assist dental professionals make the best possible selection, the FDA requires that dental burs be paired with labels conveying critical details of type, length, width, shape and other characteristics. Moreover, thorough warnings and guidelines as to how the bur should be used must also be conspicuously presented on the label. In this way, the FDA hopes to prevent any liabilities for mishandling or improper usage.
Strict regulations imposed by the FDA guarantee that any dental burs used today are a result of careful testing for safety and efficacy. To achieve this, these products are submitted for assessment by renowned independent laboratories, satisfying the exceedingly high criteria established by the organisation. Furthermore, operational procedures in manufacturing sites are regularly inspected by the FDA to guarantee hygienic and secure production of dental burs.
By registering with the FDA, dental bur manufacturers submit details of their manufacturing sites and operations to guarantee the safety and quality of their products are in line with good production practices. The agency relies on this data to ensure patients experience the best possible care.
To guarantee maximum protection for patients, the FDA has imposed rigorous limits on the concentration of lead and other heavy metals that are permissible in dental burs. This helps to ensure that dental tools are suitable and safe for use in dental treatment.
The FDA provides dental burs with an added seal of safety in the form of a “CE” mark. This label demonstrates that it has undergone necessary assessment and is deemed fit for use in dental operations.
Strict FDA regulations and guidelines are in place to guarantee the safety of dental burs and other tools used in dentistry. As a result, manufacturers, dentists, and dental staff can be confident that their dental instruments can be safely applied in dental procedures.
Dentistry requires a variety of tools, none of which plays a more significant role than dental burs. These instruments, employed by experienced dental professionals, are used to cut, craft, and gloss materials ranging from tooth enamel to denture acrylics. Moreover, they are employed in the removal of decay and dentin as part of the process of restoring a broken or damaged tooth.
In the United States, dental burs are given special governing attention courtesy of the Food and Drug Administration (FDA). According to the Medical Device Amendments of 1976, burs must be judged on safety, efficacy, and have clear labelling and precise instructions for their proper use. Furthermore, any bur used for medical diagnosis, prevention or treatment is doubly subject to FDA regulation.
Different dentists may favor various sizes, shapes, and materials of dental burs, as each has its own unique features and bonus points. Stainless steel burs are strong and resilient, yet tricky to handle. Tungsten carbide burs, although pricier, are more accurate and controllable. As for the most exacting and toughtest option available, diamond-coated burs are a perfect fit, with the expensive price tag to be expected.
When assessing the requirements for purchasing dental burs, it is essential to bear in mind the regulations laid down by the FDA. Every bur must disclose its maker’s details in terms of address, contact information and printing of the date of its fabrication. Furthermore, in compliance with FDA guidelines, no visible defects should be present in the bur and it must also remain sealed until ready for use.
It is crucially essential that burs be utilized according to the directions from their maker. Prior to any use, they must also undergo the right form of sterilization. Imprudent sterilization may result in contamination, so it is highly important only to use processes and products that have been approved by the FDA.
To maintain dental health and comply with the FDA’s regulations, proper use of burs is essential. Cleaning and disinfecting them between usages is vital, as is washing one’s hands before and after every application. Moreover, it is necessary to take care to avoid contact with the bur and the patient’s exposed skin or mucous membranes.
Consistently checking for signs of deterioration – such as chips, cracks, and dulling – in burs is vital. If their performance is diminished due to wear and tear, they should be replaced accordingly. If any bur proves faulty or substandard, it should be returned to the provider for a full replacement.
If dentists comply with the FDA’s protocols and maintain excellent oral hygiene, dental burs should be employed in a secure and proficient manner. This will guarantee patients obtain the greatest dental services possible.
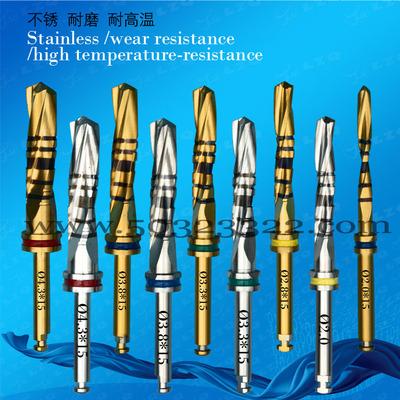
Related Product

Supply Roland DLC Zirconia Burs
Product Information Origin Tianjing, China Series Dental Bur Brand MSK Cutting Edge Form 2 Blade/3 Blade Ball Diameter (Mm) 0.6, 1, 2 Material Very Fine Grained Cemented Car […]

Diamond Coating Round Diamond Cutters
Product Information Origin Tianjing, China Series U Series Brand MSK Cutting Edge Form Helical Structure Ball Diameter (Mm) 3 Material Carbide Minimum Cutting Diameter At Th […]
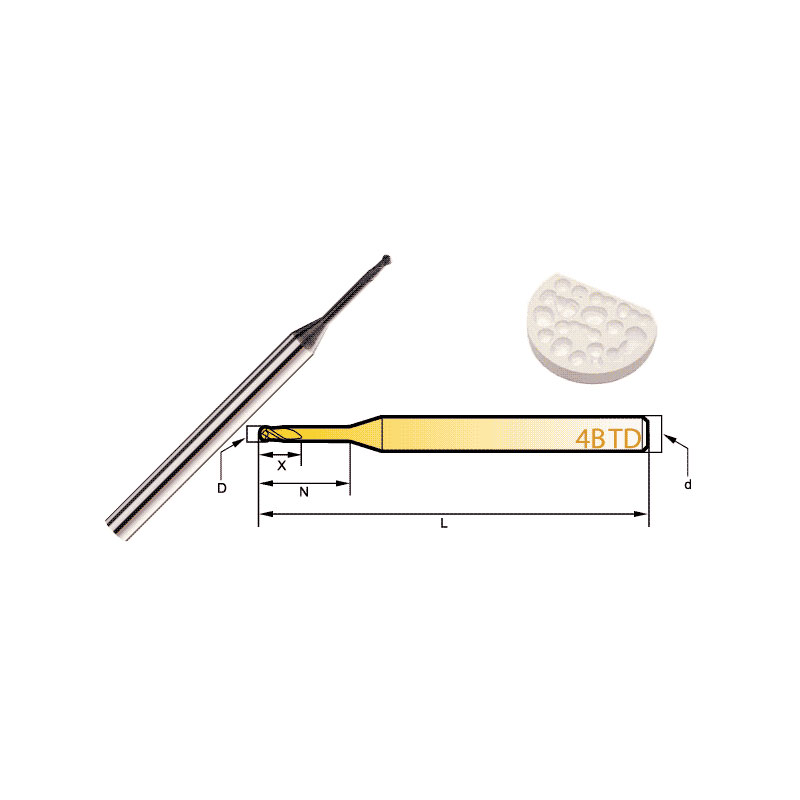
Diamond Bur Ball Round
Product Information Product Name Dental 4-Flute Ball End Mill Brand MSK Model D Number Of BladesZ X N L d 4BTD2060 2 4 6 6 50 3 4BTD2010 2 4 6 10 50 3 4BTD2016 2 4 6 […]

Step Bur Milling Bur Grinder for Glass Cerami
Product Information Origin Tianjing, China Shank Diameter 1.8 (mm) Brand MSK Scope Of Application CEREC3 Grinding Equipment Material Stainless Steel/Carbide Main Sales Areas […]

Dental CAD/CAM Milling Burs
Product Information Origin Tianjing, China Material Stainless Steel Brand MSK Applicable Machine Tools A Variety Of Options Custom Processing Yes Whether To Coat No Is It a […]
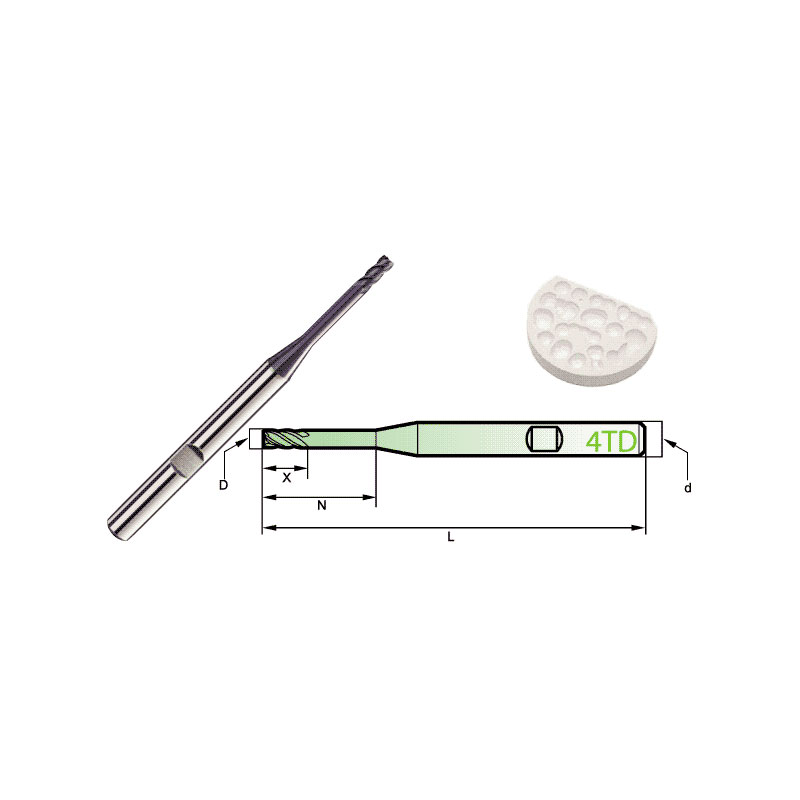
Carbide Roland CAD/CAM Burs
Product Information Origin Tianjing, China Brand MSK Number Of Blades 4 Product Name Dental Special 4-Blade End Mill Model D Number Of Blades Z X N L d 4TD2060HB 2 4 […]

HP Deburring Carbide Burs
Product Information Brand MSK Material Tungsten Steel Model Grinding Head Custom Processing Yes Feature: The dental grinding head is made of tungsten steel with stabl […]
Post time: 2023-07-27
